Background
Unlike most solid tumors, the incidence of TP53 aberrations in ALL is only 3% at diagnosis. However, in relapsed ALL this number increases to 12%. More importantly, at relapse, loss of p53 function is associated with therapy failure, with an overall survival of only 20%. Hence, there is a clear clinical need for more effective strategies to treat TP53 aberrant relapsed ALL. Owing to their high proliferative state, leukemic blasts show a high dependency on pyrimidines for DNA synthesis. A lack of pyrimidines leads to replication stress, causing activation of p53 and the DNA damage response pathway.
Results
We used CRISPR/Cas9 to generate TP53 deficient variants of the TP53 proficient BCP-ALL cell lines Nalm6 and RCH-ACV. We used these cell lines in a CRISPR screens and high throughput drug screen, and identified a strong synergy between the targeting of pyrimidine synthesis and DNA checkpoint kinase ATR and its downstream effectors Chk1 and Wee1 in TP53 deficient ALL. To validate these results, we tested the efficacy of a checkpoint inhibition together with Orludodstat (BAY-2402234), an inhibitor of Dihydroorotate Dehydrogenase (DHODH), a key enzyme in de novo pyrimidine biosynthesis. This clinical grade inhibitor was evaluated in either wild type or TP53 deficient BCP-ALL and T-ALL cell line models and patient derived xenografts (PDX).
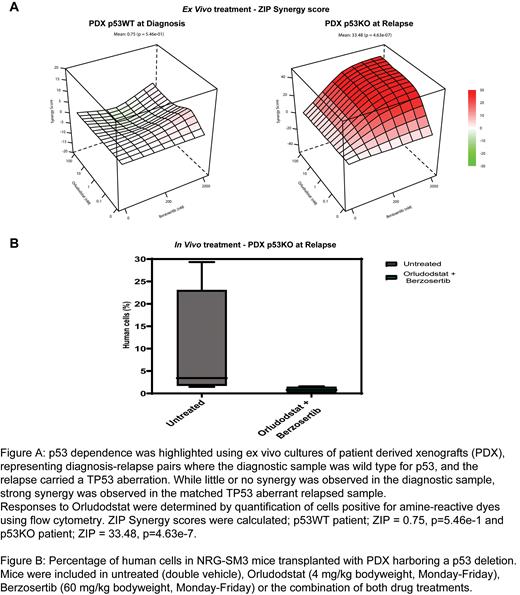
A strong apoptotic response was observed when Orludodstat was combined with the ATR inhibitor Berzosertib, most notably in TP53 deficient ALL. This p53 dependency was confirmed using ex vivo cultures of patient derived xenografts (PDX) of diagnosis and relapse pairs, where each diagnostic sample was wild type for TP53, and the relapse carried a TP53 aberration. While little or no synergy was observed in the diagnostic samples, a strong efficacy of the combination was observed in the matched TP53 deficient relapsed samples (Figure 1 A).
Preliminary results from i n vivo experiments using p53 deficient patient derived xenografts indicate that the combination of Orludodstat and Berzosertib is well tolerated, while significantly delaying leukemic growth (Figure 1 B).
Conclusion
Together, our findings suggest that the combined targeting of de novo pyrimidine synthesis and ATR signaling may present a specific and non-genotoxic vulnerability for TP53 aberrant ALL.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal